The severity of asthma can be graded from mild intermittent disease, with little impact on everyday life, through to severe disease with permanent symptoms and serious limitation of normal activities.
Patients with severe asthma can be broadly classified as eosinophilic (Th2-high) or non-eosinophilic (Th2-low) phenotypes; subpopulations with distinct pathologies, clinical characteristics and treatment management strategies. Despite numerous drugs now being marketed for the treatment of severe asthma (i.e. monoclonal antibody therapies targeting interleukin (IL)-5 and IL-4/IL-13 cytokine pathways), an unmet need persists for many patients. This includes severe non-eosinophilic asthmatics, for whom no targeted therapies are currently available, as well as severe eosinophilic asthmatics with sub-optimal response to anti-interleukin therapies.
Masitinib is currently being investigated in severe asthma uncontrolled by oral corticosteroids (OCS) and also in severe asthma uncontrolled by high dose inhaled corticosteroids (ICS).
Masitinib is a first in class oral drug in severe asthma, selectively targeting mast cells and is uniquely positioned in this indication in terms of administration (oral administration), mechanism of action, targeted population, and broad eosinophil level.
The number of patients targeted by masitinib amounts to 825,000 in Europe and in the US.
In this phase 2a study, a comparable reduction in oral corticosteroids was achieved with masitinib and placebo at 16 weeks of treatment (median reduction of 78% and 57% in the masitinib and placebo arms, respectively). However, when focusing on the truly dependent corticosteroids asthma patients, meaning patients receiving > 15mg equivalent prednisone per day, a significant difference was observed. In this subgroup of patients, doses of corticosteroids were reduced by 52% in masitinib treated patients compared to 28% in placebo treated patients.
The results of this study have been published in the European Journal of Allergy and Clinical Immunology.
AB Science reported positive phase 3 study results in severe asthma uncontrolled by oral corticosteroids (OCS). In this study, masitinib significantly decreased the rate of severe asthma exacerbations in a population that was comprised of both eosinophilic (Th2-high) and non-eosinophilic (Th2-low) severe asthmatics.
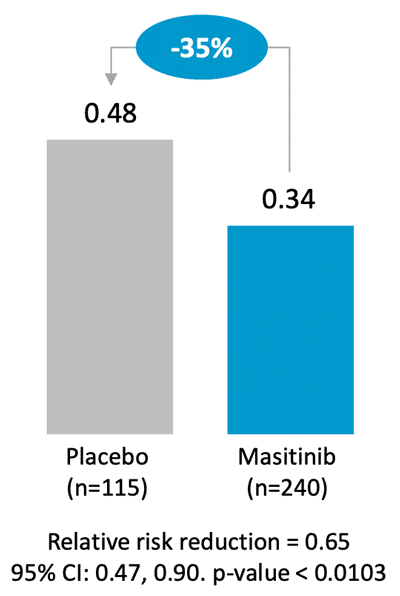
Primary analysis was conducted in the severe asthma population with daily OCS ≥ 7.5 mg and masitinib treatment was associated with a significant reduction in severe asthma exacerbations (-35%). A pre-specified subgroup of severe asthma patients with baseline blood eosinophil count of ≥ 150 cells/μL also demonstrated a statistically significant reduction in rate of severe asthma exacerbations (-38%).
Primary analysis – Severe asthma exacerbations
The results of the study have been presented at the American Thoracic Society (ATS) 2020 International Conference in May 2020, at the European Academy of Allergy & Clinical Immunology (EAACI) 2020 Congress in June 2020 and at the 30th annual European Respiratory Society (ERS) International Congress in September 2020.
AB Science also reported positive phase 3 study results in severe asthma uncontrolled by high-dose inhaled corticosteroids (ICS) and with eosinophil level >150 cells/μL. The pre-specified primary analysis was rate of severe asthma exacerbations, with masitinib demonstrating a statistically significant 29% reduction in severe exacerbations relative to placebo (p=0.022). The frequency of severe asthma exacerbations was 0.43 in the masitinib arm, versus 0.62 in the placebo arm.