If you are interested in participating in the ongoing study, please contact us at clinical@ab-science.com
Amyotrophic lateral sclerosis (ALS) is a rare and life-threatening neurodegenerative disease characterized by progressive muscular paralysis reflecting degeneration of motor neurons. ALS is associated with substantial morbidity and severely impacts day-to-day functioning. The disease is relentlessly progressive.
There is still a high need for new treatments in ALS. In Europe, there has been no drug registered since riluzole 32 years ago.
Masitinib is currently the only tyrosine kinase inhibitor in late-stage development for ALS. Masitinib distinguishes itself from other ALS developmental drugs by exerting neuroprotection in both central and peripheral nervous systems.
Masitinib appears exceptional among other ALS-developmental drugs, exerting neuroprotection in both central nervous system and peripheral nervous system via selective kinase inhibition that modulates the functionality of different cells implicated in ALS pathogenesis.
In recognition of the critical need for new treatments, masitinib received orphan drug designation for ALS from both the European Medicine Agency (EMA) and the U.S. Food and Drug Administration (FDA).
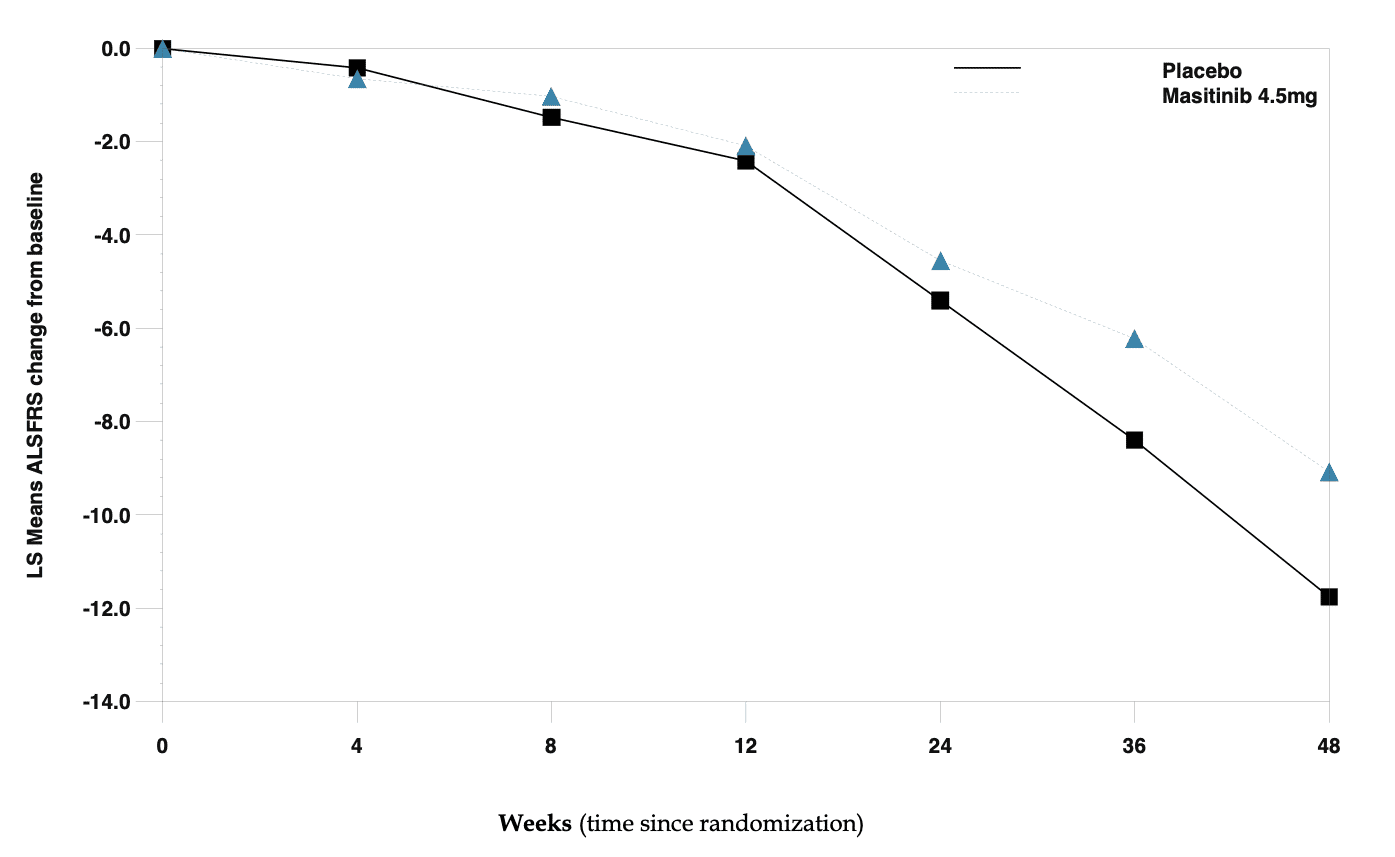
AB Science reported positive Phase 2B/3 results with masitinib in ALS. In this study, masitinib in combination with riluzole demonstrated a significant delay (-27%) in disease progression in ALS patients identified as normal progressors, which was the population for primary analysis (i.e. patients with a baseline ALSFRS-R progression of <1.1 point/month).
Primary analysis – Change in ALSFRS score from baseline
The results of the study have been published in the journal Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration (ALSFD) and represent the first positive phase 3 trial of a tyrosine kinase inhibitor in ALS.
A survival analysis followed all patients originally randomized in study AB10015 for an average duration of 75 months from the date of diagnosis. In ALS patients with mild or moderate disease severity at baseline, it was seen that treatment with 4.5 mg/kg/day masitinib as an add-on to standard riluzole prolonged survival by 25 months relative to those treated with riluzole alone, with a 44% reduced risk of death. Patients with mild or moderate disease severity correspond closely to the patient cohort enrolled in confirmatory phase 3 study, AB19001. This new survival data has been published in the peer-reviewed journal Therapeutic Advances in Neurological Disorders.
Finally, upon further analysis, it appeared that there is a clear increased benefit in the subgroup referred to as “ALS prior to any complete loss of function” (i.e. excluding patient with an ALSFRS-R score of 0 in any of the 12 items of the scale). Indeed, a significant + 12 months survival benefit (p=0.0192) was observed in this subgroup.
A new confirmatory Phase 3 study, simplified for enrolment and targeting best responders for masitinib will be initiated in line with recommendation of FDA and EMA. The design of the phase 3 confirmatory study has recently been approved by FDA and EMA (Clinical Trials Information System (CTIS) Step 1).
