Multiple sclerosis (MS) is a disease of the central nervous system (CNS) characterized by a progressive degradation of CNS nerve cells by the patient’s immune system and comes in two main forms : the relapsing-remitting form characterized by relapses of the disease and the progressive form, characterized by a constant and regular worsening of the symptoms of the disease, without a distinct relapse or period of recovery.
While significant progress has been made in the relapsing form of MS, with more than 15 approved drugs, there is still a very high unmet medical need for treating patients with primary progressive MS (PPMS) and non-active secondary progressive MS (nSPMS), with no approved drugs for nSPMS and only one for PPMS.
In progressive multiple sclerosis, innate immune cells such as macrophages, microglia or mast cells have been shown to probably play a major role. Masitinib is designed for progressive forms of multiple sclerosis, targeting the innate immune system, specifically mast cells and microglia.
This phase 2a study showed that for the primary endpoint of MSFC (which measures symptoms of patients on three aspects: movement of the lower limbs, movement of the upper limbs, and cognitive tests), 32% of patients treated with masitinib were responders as compared with 0% with placebo. A clinical response was defined as > 100% change from baseline in MSFC score.
Results of this Phase 2a study have been published in BMC Neurology.
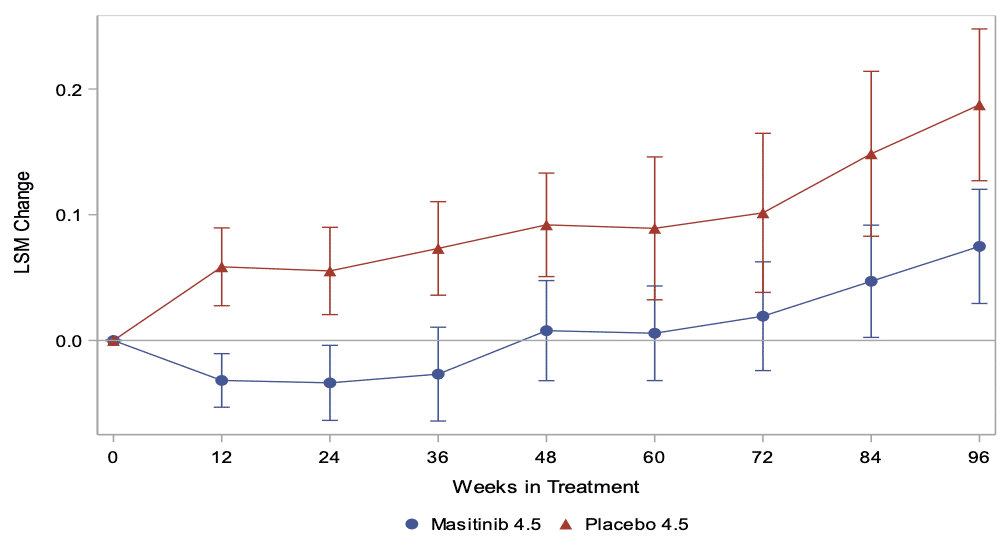
AB Science reported positive Phase 2B/3 results with masitinib in progressive forms of MS. In this study, masitinib slowed down disease progression in patients, which was the study’s primary objective, as measured by the change on the Expanded Disability Status Scale (EDSS). Masitinib also demonstrated a significant reduction in the risk of reaching a level of disability severe enough to require wheelchair mobility.
Primary analysis – Change in EDSS up to week 96 (Positive value indicates worsening)
The results of the study have been presented at the 8th Joint Meeting of the European (ECTRIMS) and American (ACTRIMS) Committees for Treatment and Research in Multiple Sclerosis (MSVirtual2020) and provides the first clinical evidence that targeting the innate immune system is an effective strategy for the treatment of progressive forms of multiple sclerosis.
AB Science received authorization to initiate a confirmatory phase III study evaluating masitinib in patients with Primary Progressive Multiple Sclerosis (PPMS) or non-active Secondary Progressive Multiple Sclerosis (nSPMS). The study will enroll 800 patients from numerous study centers with Expanded Disability Status Scale (EDSS) score between 3.0 to 6.0 and absence of T1 Gadolinium-enhancing brain lesions as measured by magnetic resonance imaging (MRI). The primary objective of the study will be to evaluate the effect of masitinib on time to confirmed disability progression, with progression defined as 1-point worsening when EDSS baseline score ≤5.5, or 0.5 if baseline score >5.5 from randomization to week 96.